Recall: Sandoz and Novartis Recall Prescription Drug Blister Packages Due to Failure to Meet Child-Resistant Closure Requirements
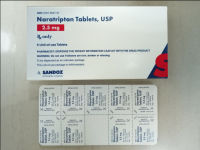
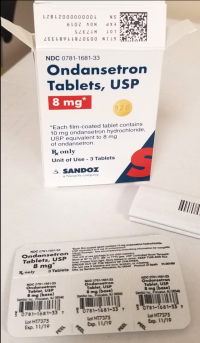
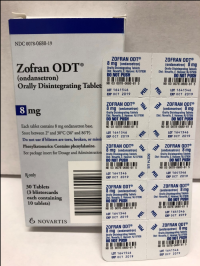
Description: This recall involves blister packages of prescription drugs from Novartis and Sandoz. The drugs are packaged with 3 to 10 tablets per blister card. The recalled Novartis prescription blister packages have "Novartis," the name of the drug, dosage, NDC, lot number and expiration date printed on the cartons and the blister cards. The recall includes the following: Recalled Novartis Prescription Drugs NDC Numbers Tablet Strength Carton Configuration Lot Numbers Expiration Date Zofran ODT® 0078-0679-61 0078-0679-19 4 mg 30 count: 3 cards with 10 tablets each 1657088 Dec 2019 Zofran ODT® 0078-0680-61 0078-0680-19 8 mg 30 count: 3 cards with 10 tablets each 1641546 Oct 2019 Entresto® (sacubitril/valsartan) 0078-0659-61 0078-0659-35 24 mg/ 26 mg 100 count: 10 cards with 10 tablets each FX000005 FX000004 FX000003 F0010 F0009 F0007 Apr 2020 Apr 2020 Sep 2019 Nov 2018 Aug 2018 Jul 2018 Entresto® (sacubitril/valsartan) 0078-0777-61 0078-0777-35 49 mg/ 51 mg 100 count: 10 cards with 10 tablets each FX000001 F0006 F0005 F0004 Dec 2019 Oct 2019 Aug 2019 Oct 2018 Entresto® (sacubitril/valsartan) 0078-0696-61 0078-0696-35 97 mg/ 103 mg 100 count: 10 cards with 10 tablets each FX000002 F0007 F0006 F0005 F0004 Mar 2020 Feb 2020 Dec 2019 Dec 2018 Oct 2018 The recalled Sandoz prescription blister packages have "Sandoz," the name of the drug, dosage, NDC and lot number printed on the cartons and the blister cards. Lot numbers are listed at www.us.sandoz.com/patients-customers/product-safety-notices. The recall includes the following: Recalled Sandoz Prescription Drugs Tablet Strength NDC Numbers Carton Configuration Azithromycin Tablets 250 mg 0781-5776-06 0781-5776-69 50 count: 5 cards with 10 tablets each Donepezil ODT Tablets 5 mg 0781-5276-06 0781-5276-64 30 count: 3 cards with 10 tablets each Donepezil ODT Tablets 10 mg 0781-5277-06 0781-5277-64 30 count: 3 cards with 10 tablets each Haloperidol Tablets 0.5 mg 0781-1391-13 100 count: 10 cards with 10 tablets each Haloperidol Tablets 1 mg 0781-1392-13 100 count: 10 cards with 10 tablets each Haloperidol Tablets 2 mg 0781-1393-13 100 count: 10 cards with 10 tablets each Haloperidol Tablets 5 mg 0781-1396-13 100 count: 10 cards with 10 tablets each Haloperidol Tablets 10 mg 0781-1397-13 100 count: 10 cards with 10 tablets each Imipramine HCl Tablets 25 mg 0781-1764-13 100 count: 10 cards with 10 tablets each Imipramine HCl Tablets 50 mg 0781-1766-13 100 count: 10 cards with 10 tablets each Isosorbide Dinitrate (ISDN) Tablets 10 mg 0781-1556-13 100 count: 10 cards with 10 tablets each Isosorbide Dinitrate (ISDN) Tablets 20 mg 0781-1695-13 100 count: 10 cards with 10 tablets each Naratriptan Tablets 2.5 mg 0781-5527-06 0781-5527-37 9 count: 1 card with 9 tablets Ondansetron Tablets 8 mg 0781-1681-33 3 count: 1 card with 3 tablets Ondansetron ODT 4 mg 0781-5238-06 0781-5238-64 30 count: 3 cards with 10 tablets each Ondansetron ODT 8 mg 0781-5239-06 0781-5239-64 30 count: 3 cards with 10 tablets each Ondansetron ODT 8 mg 0781-5239-06 0781-5239-80 10 count: 1 card with 10 tablets Perphenazine Tablets 2 mg 0781-1046-13 100 count: 10 cards with 10 tablets each Perphenazine Tablets 4 mg 0781-1047-13 100 count: 10 cards with 10 tablets each Perphenazine Tablets 8 mg 0781-1048-13 100 count: 10 cards with 10 tablets each Risperidone ODT 0.5 mg 0781-5310-06 0781-5310-08 28 count: 7 cards with 4 tablets each Risperidone ODT 1 mg 0781-5311-06 0781-5311-08 28 count: 7 cards with 4 tablets each Risperidone ODT 2 mg 0781-5312-06 0781-5312-08 28 count: 7 cards with 4 tablets each Risperidone ODT 3 mg 0781-5313-06 0781-5313-08 28 count: 7 cards with 4 tablets each Risperidone ODT 4 mg 0781-5314-06 0781-5314-08 28 count: 7 cards with 4 tablets each
Name of product: Blister packages of prescription medication
Units: About 470,000
Hazard: The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act, posing a poisoning risk if swallowed by children.
Remedy: Consumers should immediately secure the blister cards to keep them out of the sight and reach of children and contact Novartis or Sandoz for further instructions. Novartis and Sandoz advise that consumers should continue to use the medication as directed once the blister packages are secured.
Recall date: July 06, 2018
Incidents/Injuries: The firms have received one report of a child ingesting haloperidol from a blister pack.
Sold At: Clinics and pharmacies nationwide as a prescribed medicine from September 2016 to June 2018, at prices varying based on quantities prescribed, health insurance terms and other factors.
Manufactured in: Croatia, India, Ireland, Israel, Italy, Singapore, Spain, United Kingdom, United States
Consumer contact: Sandoz and Novartis toll-free at 888-669-6682 from Monday to Friday, 8 a.m. to 8 p.m. ET and Saturday and Sunday, 9 a.m. to 6 p.m. ET or online at www.us.sandoz.com and click on “Patients and Customers” then “Product Safety Notices,” or at www.pharma.us.novartis.com and click on banner “Novartis recalls select product blister packs.”
Recall number: 18-183