Recall: Sandoz Recalls Aprepitant Capsules and Lidocaine and Prilocaine Cream Prescription Drugs Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning
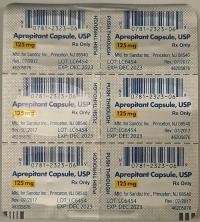
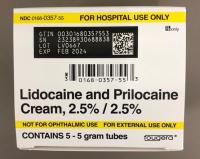
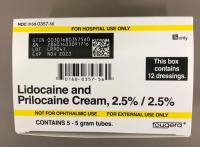
Description: This recall involves prescription drugs Aprepitant 125 mg capsules sold in cartons containing one blister card of 6 capsules and 5 gram tubes of Lidocaine and Prilocaine cream sold in cartons containing 5 tubes and packed with or without 12 dressings. The Aprepitant capsules are in a non-child resistant blister card packaged in a carton that has the name "Sandoz," the name of the medication, dosage, NDC number, lot number, and expiration date on the carton and on the blister cards. The warnings "This unit-dose packaging is not child-resistant" and "For institutional use only" are listed on the carton. The Lidocaine and Prilocaine cream is packaged in a 5 gram tube with a continuous thread white closure. The name "fougera®," the name of the medication, dosage and NDC number are printed on the carton and tube and the expiration date and lot number are printed on the carton and stamped on the crimp of the tube. The warning "FOR HOSPITAL USE ONLY" is printed on the carton and the tube. Product Description NDC Number Lot Number Expiration Date Aprepitant Capsules 125 mg 0781-2323-68 Carton of 1 Blister Pack of 6 capsules 0781-2323-06 Blister Pack LK3209 LC6454 04/2024 12/2023 Lidocaine and Prilocaine 2.5%/2.5% Cream 5 gram Tubes 0168-0357-56 Carton of 5 tubes and 12 dressings 0168-0357-55 Carton of 5 tubes 0168-0357-05 Tube LA2782 LA2784 LV0667 LX5350 MA1640 MB3205 LA2785 LR9041 MB3209 03/2023 03/2023 02/2024 03/2024 03/2024 04/2024 03/2023 11/2023 04/2024
Name of product: Aprepitant capsules and Lidocaine and Prilocaine cream
Units: About 156,750
Manufacturer: Sandoz Inc., of Princeton, New Jersey (Lidocaine and Prilocaine cream)
Hazard: The recalled prescription drugs and products that contain lidocaine must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
Remedy: Consumers should immediately secure the medications out of the sight and reach of children and contact Sandoz for a free child resistant pouch to store the products. Once the medication is secured, consumers can continue to use the medication as directed.
Recall date: March 09, 2023
Incidents/Injuries: None reported
Sold At: Pharmacies nationwide as a prescribed medicine from October 2020 through January 2023. The prices of the medications varied based on health insurance terms and other factors.
Importers: Sandoz Inc., of Princeton, New Jersey (Aprepitant Capsules)
Manufactured in: Slovenia (Aprepitant Capsules), United States (Lidocaine and Prilocaine cream)
Consumer contact: Sandoz toll-free at 866-300-2207 from 8 a.m. to 5 p.m. ET Monday through Friday, email at Sandoz6768@sedgwick.com or online at https://www.us.sandoz.com/patients-customers/product-safety-notices or www.us.sandoz.com and click on "Product Safety Notices" below the carousel for more information.
Recall number: 23-146