Recall: YYBA Recalls Welmate Lidocaine Numbing Cream Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning (Recall Alert)
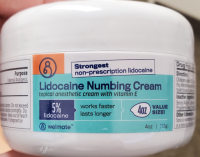
Description: This recall involves Welmate Lidocaine Numbing Cream in a 113 g (4 oz.) white jar with a blue, orange and green label and a white cap. "Welmate," "5% Lidocaine," the Welmate logo and UPC code 373581105045 or 373581000012 are printed on the label. The lot numbers and corresponding expiration dates included in the recall are printed on the underside of the jar as follows: Lot Numbers Expiration Dates ELNC2001 05/2022 ELNC2002 05/2022 EL5C2001 11/2022 EL5C2002 11/2022 EL5C2101 11/2022 EL5C2102 11/2022
Name of product: Welmate Lidocaine Numbing Cream
Units: About 15,000
Manufacturer: Yash Pharmaceuticals, of India
Hazard: The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain relieving cream contains lidocaine, posing a risk of poisoning to young children if they put it on their skin or ingest it.
Remedy: Consumers should immediately stop using the recalled cream, store it in a safe location out of reach of children and contact YYBA for instructions on how to dispose of the product to receive a full refund. YYBA is directly notifying all known consumers who purchased the recalled product on eBay, Walmart and wellspringmeds.com. Amazon is directly notifying all customers who purchased the recalled product on Amazon.com.
Recall date: April 22, 2021
Incidents/Injuries: No incidents or injuries have been reported.
Sold At: Online at Amazon.com, Ebay.com, Walmart.com and wellspringmeds.com from August 2020 through March 2021 for about $20.
Importers: One2Zee LLC, of Monroe, N.J.
Manufactured in: India
Consumer contact: YYBA toll-free at 866-933-6337 from 10 a.m. to 6 p.m. ET Monday through Friday, email at recall@wellspringmeds.com or online at www.wellspringmeds.com and click on "Recall - Important Safety Information" for more information.
Recall number: 21-737