Recall: Women's Iron Complete Supplements Recalled by GNC Due to Failure to Meet Child Resistant Closure Requirement; Risk of Poisoning
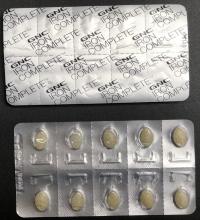
Description: This recall involves Women's Iron Complete Dietary Supplement 60-count caplets. The recalled dietary supplement is in a white box with "Women's Iron Complete" printed on the front in gray and red font. The box contains blister packets with a total of sixty caplets.
Name of product: Women's Iron Complete Dietary Supplement (60 caplets)
Units: About 756,000
Manufacturer: Nutra Manufacturing, Inc., of Greenville, S.C. (a GNC Company)
Hazard: The dietary supplement blister packaging is not child resistant, as required by federal law. If ingested by a child, these supplements could cause serious injury or death.
Remedy: Consumers should keep these products out of the reach of children and contact GNC for instructions on how to obtain a refund. Consumers can return the unused product to their local GNC store for a refund.
Recall date: December 19, 2018
Incidents/Injuries: None reported
Sold At: GNC retail stores nationwide and online at www.gnc.com from September 2000 through August 2018, and online at www.drugstore.com from September 2000, through August 2016 for about $10.
Manufactured in: United States
Consumer contact: GNC at 888-462-2548 any time, email at customer-service@gnc-hq.com or online at www.gnc.com and click on “Recall Notice” link at the top of the page for more information.
Recall number: 19-055